



安心・安全な商品の提供


種子・畑から安全な農産原料づくりに取り組み、設計開発・調達・生産・物流・販売の各工程でカゴメ品質マネジメントシステム(KQMS)を回し、安心・安全な商品の提供に努めています。
品質・環境方針
カゴメグループの事業活動の継続のためには、豊かな自然環境のもとでの持続的な農業の営みが欠かせず、自然を活かしたものづくりを保証する体制と、地球環境の保全を両立させていくことが必要不可欠です。
「カゴメグループが情熱を込めて取り組んできたものづくりと同じ想いで環境保全にも注力することで、持続可能な社会の実現を目指す」という経営の意志を込め、従来の「品質方針」と「環境方針」を統合し、2017年10月に「品質・環境方針」を制定しました。
1. 野菜によるおいしさと健康価値で、大切な人の健康長寿に貢献します。
2. 国内外のパートナーと種子・畑から一貫した安全な農産原料づくりに取り組みます。
3. 野菜を育む水・土・大気を守り、豊かな自然をつくる農業を未来につなげ、得られた恵みを有効に活用します。
4. 法令や自主基準を順守し、しくみや行動をレベルアップし続けることで、安全で環境に配慮した商品をお客様にお届けします。
5. お客様へ商品やサービスの確かさをお伝えしつつ、お客様の声を企業活動へ反映します。
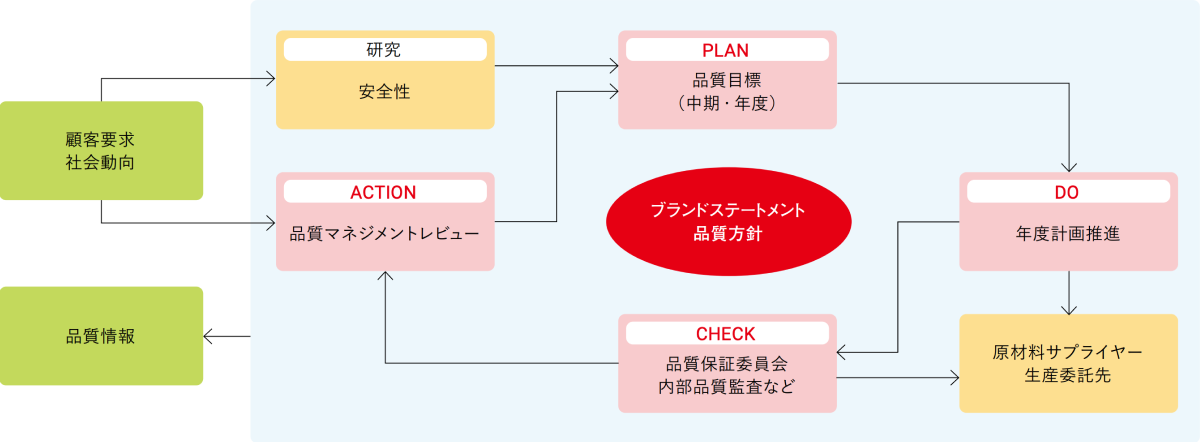
カゴメ品質マネジメントシステム(KQMS)
カゴメグループには「品質第一・利益第二」という考え方があります。これはお客様に安心・安全な品質を提供することと、利益の創出をどちらも大事にするという考え方です。品質を保証する体制として、国際規格ISO9001に準拠した独自の品質マネジメントシステム(Kagome Quality Management System:KQMS)を構築し、設計開発から調達・生産・物流・販売にわたる品質活動に取り組んでいます。

安心・安全のブランド力
ブランド戦略サーベイ2024(株式会社日経リサーチ)
・製品・サービスの品質が高いブランド2位(食品企業では1位)
・安全で間違いのない品質を得られるブランド10位(食品企業では4位)
【調査概要】
※使用データ コンシューマー編
※測定対象社数:600社
※調査期間:2024年6~7月
※回答者数:1企業につき約750名
※調査対象:日経リサーチ・提携協力会社インターネットモニター登録の全国の16歳以上の方





